10.06.2016
0800
Processing Alkaline Batteries Link
Recycling Lithium Batteries Link
I question the honesty of how items are recycled (especially electronics) and to be more informed is always better. The next series of posts I’m going to post up with cover a small section of the majority of materials that are deemed recyclable. Although the concept of recycling seems like a savior process for all items- it really isn’t. There are uncomfortable truths that the public is not informed about. I hope these next posts will be helpful for those who are seeking more information.
In a nutshell, batteries vary in how they are recycled. Batteries range from lead acid based to alkaline, lithium ion, nickel, zinc and even mercury batteries. Here is an overall information haul about the variety of them but I also included links to some recycling processes under the infographics above. I always hear mixed reviews as to what actually happens to batteries when we recycle them and this is why I thought I should post some information. I tend to use more alkaline and lithium batteries in my day to day life, so those infographics apply more to me. Hopefully this will bring some more information to you as you come into contact with your day to day electronics that use batteries.
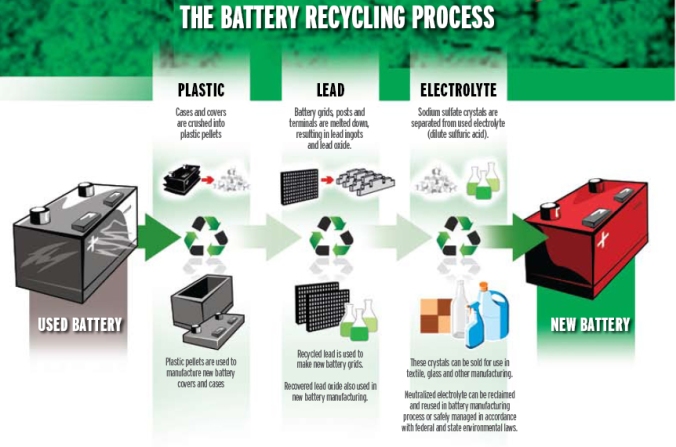
- Lead Acid– The battery is broken apart in a hammer mill, a machine that hammers the battery into pieces. The broken battery pieces are then placed into a vat, where the lead and heavy materials fall to the bottom and the plastic floats. At this point, the polypropylene pieces are scooped away and the liquids are drawn off, leaving the lead and heavy metals. Each of the materials goes into a different recycling “stream”.
- Plastic- Polypropylene pieces are washed, blown dry and sent to a plastic recycler where the pieces are melted together into an almost liquid state. The molten plastic is put through an extruder that produces small plastic pellets of a uniform size. The pellets are put back into manufacturing battery cases and the process begins again.
- Lead- Lead grids, lead oxide and other lead parts are cleaned and heated within smelting furnaces. The molten melted lead is then poured into ingot molds. After a few minutes, the impurities float to the top of the still molten lead in the ingot molds. These impurities are scraped away and the ingots are left to cool. When the ingots are cool, they’re removed from the molds and sent to battery manufacturers, where they’re re-melted and used in the production of new batteries.
- Sulfuric Acid- Old battery acid can be handled in two ways:
- The acid is neutralized with an industrial compound similar to household baking soda. Neutralization turns the acid into water. The water is then treated, cleaned, tested in a wastewater treatment plant to be sure it meets clean water standards.
- The acid is processed and converted to sodium sulfate, an odorless white powder that’s used in laundry detergent, glass and textile manufacturing.Lead acid batteries are closed-loop recycled, meaning each part the the old batteries is recycled into a new battery. It is estimated that 98% of all lead acid batteries are recycled.
-
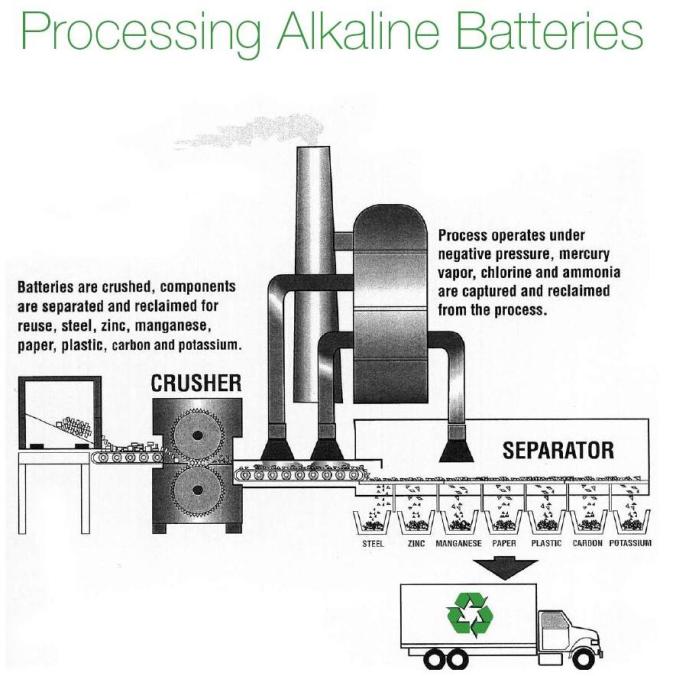
Alkaline batteries– Alkaline batteries such as (AAA, AA, C, D, 9V, etc.) are recycled in a specialized “room temperature,” mechanical separation process where the battery components are separated into three end products. These items are a zinc and manganese concentrate, steel, and paper, plastic and brass fractions. All of these products are put back into the market place for reuse in new products to offset the cost of the recycling process. These batteries are 100% recycled.
-
Lithium Ion– Prior to the recycling process, plastics are separated from the metal components. The metals are then recycled via a high temperature metal reclamation (HTMR) process during which all of the high temperature metals contained within the battery feedstock (i.e. nickel, iron, manganese and chromium) report to the molten-metal bath within the furnace, amalgamate, then solidify during the casting operation. The low-melt metals (i.e. zinc) separate during the melting. The metals and plastic are then returned to be reused in new products. These batteries are 100% recycled.
-
Nickel-Cadmium- Prior to the recycling process, plastics are separated from the metal components. The metals are then recycled via a high temperature metal reclamation (HTMR) process during which all of the high temperature metals contained within the battery feedstock (i.e. nickel, iron, manganese, and chromium) report to the molten-metal bath within the furnace, amalgamate, then solidify during the casting operation. The low-melt metals (i.e. zinc and cadmium) separate during the melting. The metals and plastic are then returned to be reused in new products. These batteries are 100% recycled.
-
Nickel Metal Hydride– Prior to the recycling process, the plastics are removed from the cell portion. The cells go through a drying process to remove moisture (potassium hydroxide (KOH) electrolyte and H2O) from the cells. The drying process heats the cells in a time and temperature controlled manner via a proprietary and proven formula. Once these cells are dried they become a valuable feedstock for the stainless steel and or alloy manufacturing industries. The metals and plastic are then returned to be reused in new products. These batteries are 100% recycled.
-
Lithium Batteries– The contents of the batteries are exposed using a shredder or a high-speed hammer depending on battery size. The contents are then submerged in caustic (basic not acidic) water. This caustic solution neutralizes the electrolytes, and ferrous and non-ferrous metals are recovered. The clean scrap metal is then sold to metal recyclers to offset the cost of recycling these batteries. The solution is then filtered. The carbon is recovered and pressed into moist sheets of carbon cake. Some of the carbon is recycled with cobalt. The lithium in the solution (lithium hydroxide) is converted to lithium carbonate, a fine white powder. What results is technical grade lithium carbonate, which is used to make lithium ingot metal and foil for batteries. It also provides lithium metal for resale and for the manufacture of sulfur dioxide batteries.
-
Mercury Batteries– The batteries and heavy metals are recovered through a controlled-temperature process. It’s important to note: the percentage of mercuric oxide batteries is decreasing since the passage of the Mercury-Containing Rechargeable Battery Management Act (The Battery Act) of 1996. This act prohibits, or otherwise conditions, the sale of certain types of mercury-containing batteries (i.e., alkaline manganese, zinc carbon, button cell mercuric-oxide and other mercuric-oxide batteries) in the United States.
-
Zinc-Carbon– Zinc-carbon (AAA, AA, C, D, 9V, etc.) and zinc-air batteries are recycled in the same way as alkaline batteries or by using high temperature metal reclamation (HTMR) method to melt the metals. These metals are then reused in new products. These batteries are 100% recycled.
-
Zinc-Air– Zinc-carbon (AAA, AA, C, D, 9V, etc.) and zinc-air batteries are recycled in the same way as alkaline batteries or by using high temperature metal reclamation (HTMR) method to melt the metals. These metals are then reused in new products. These batteries are 100% recycled.
thanks for your greate share , please keep this way .
LikeLike
I like this web site very much, Its a really nice situation to read and obtain info .
LikeLike
Greetings from Colorado! I’m bored to death at work so
I decided to check out your website on my iphone during lunch break.
I enjoy the info you present here and can’t wait to take a look when I get home.
I’m amazed at how quick your blog loaded on my phone ..
I’m not even using WIFI, just 3G .. Anyways, fantastic site!
LikeLike